【8.3.4】肌红蛋白和血红蛋白
肌红蛋白(Mb)和血红蛋白(Hb)都含有血红素辅基,都能够可逆地结合氧气,都含有珠蛋白折叠这样的结构模体。
Mb存在于肌肉中,心肌含量特别丰富。其功能是为动物的肌肉组织贮备氧气,此外还能解除NO的毒性。水生哺乳动物(如鲸鱼)体内的Mb含量尤其丰富,因此它们可以在水下长时间憋气。
- 氧气在地球上的出现对于生物的进化来说十分重要,因为有氧代谢比无氧 代谢可产生更多的能量,让复杂的生命形式得以进化产生(Oxygen molecules is so crucial for the evolution of life on Earth, since aerobic metabolism yields much more energy than anaerobic metabolism).
- 然而,氧气的水溶性较差。因此,生物要想有效地利用氧气,必须找到一 种有效的运输氧的机制。而对那些高度耗氧的组织,还需要专门贮存氧气 的机制(However, oxygen has limited solubility in water, so organisms need a way to bind oxygen effectively, in order to deliver it to tissues in sufficient quantities. For those tissues which need more oxygen, there has to be a way to store oxygen).
- 血红蛋白(Hb)和肌红蛋白(Mb)就是为此进化而来,Hb 被用于在循环系统 里运输氧气,Mb专门为肌肉组织贮存氧气(Proteins like Mb and Hb evolved to fulfill these purposes. Hb is used to transport O2 in the blood stream while Mb is used to transport O2 in muscle).
为什么Mb和Hb能够结合氧气 (Why are Mb and Hb able to bind Oxygen?
它与任何氨基酸残基的侧链无关,而是与它们的血红素辅基有关 (It has nothing to do with R groups of any amino acid residues, but has something to do with their prosthetic groups-heme).

血红素辅基结合铁,铁结合氧气(The heme prosthetic group binds iron and iron binds oxygen)
铁有2个价态,但只有二价态才能结合氧气(Iron has two oxidation states: Fe2+ binds O2, while Fe3+does not bind O2).
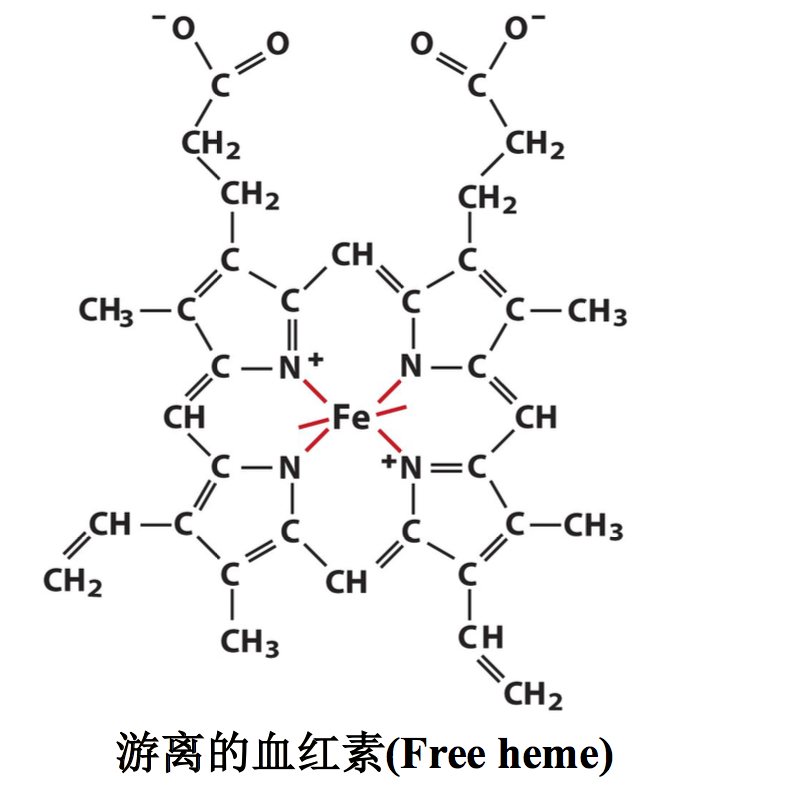
为什么生物不用游离的血红素运输或者贮存氧气(Why doesn’t Organism use free heme to transport or store O2)?
游离的血红素铁很容易被氧化成高价态,而失去结合氧气的能力。当血红素跟 血红蛋白和肌红蛋白结合以后,就被蛋白质隔离在结合部位,难以被氧化(When heme is free in solution, it can bind O2, but in the presence of a second heme molecule, O2 will oxidize Fe2+ to Fe3+, so the first heme no longer binds O2. This process continues until all of the heme iron molecules have been oxidized. One function of myoglobin and hemoglobin is to physically separate the heme molecules so oxygen does not oxidize the Fe2+) .
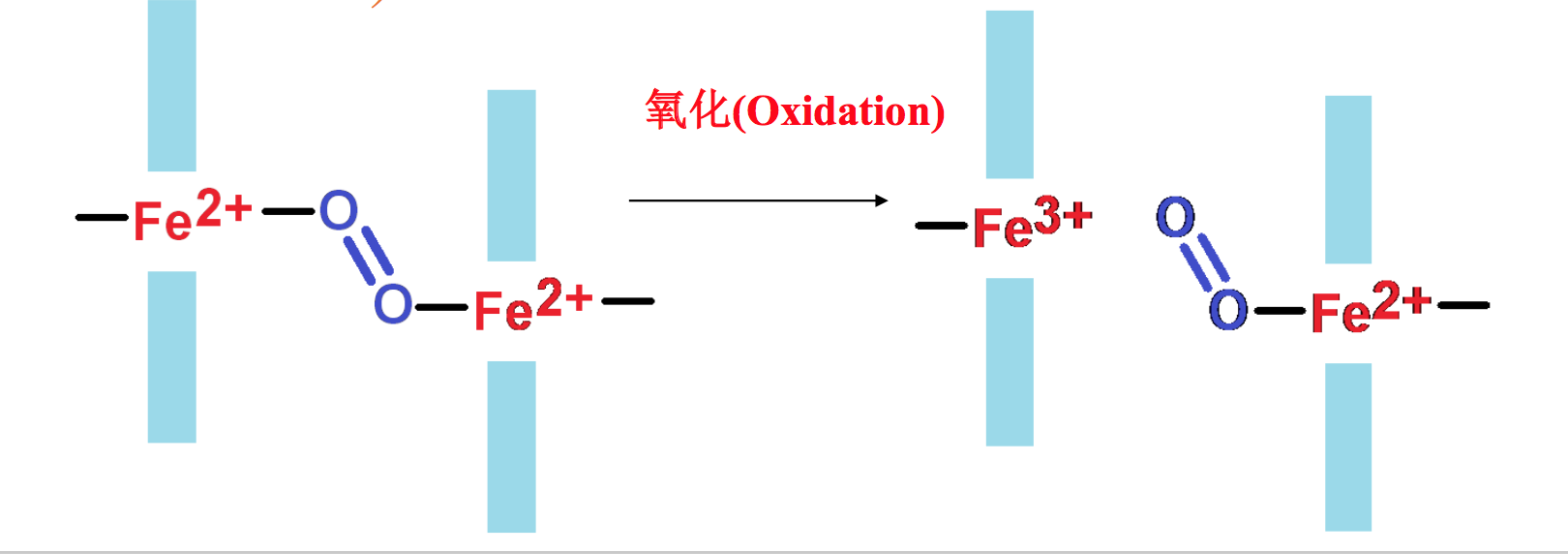
血红素铁结合在Mb和Hb上难以被氧化(The heme binding sites in Mb and Hb prevent oxidation of Fe2+ by O2)

2个高度保守的组氨酸残基对于Mb和Hb的功能非常关键(2 His residues are critical to the function of Mb and Hb)
- 1个是近端组氨酸93,它与血红素铁配位结 合,使得血红素能结合在蛋白质分子上 (One is His 93 known as the “proximal histidine”. It coordinates with the heme iron).
- 另1个是远端的组氨酸64,它的作用一是可 以防止血红素铁氧化,二是可大大降低血 红素铁与CO的亲和力(The other is His64 known as the “distal histidine”. It has two important roles: one is to prevent heme iron from oxidizing and the other is to lower affinity for binding CO by introducing a steric hindrance).

远端组氨酸降低血红素与CO的亲和力(The distal histidine reduces the affinity of heme for CO)

Mb与Hb在结构与功能上的差别 (Differences of Structure and Function between Mb and Hb)
Mb只有1条肽链组成,没有四级结构;Hb由4条肽链组成,具有四 级结构。正是这个差别,导致Hb与氧气结合具有3个效应,这3个效 应共同作用使得Hb适合充当氧气的运输者(Mb is composed of only one polypeptide chain, meaning it has no quaternary structure. Hb is composed of four polypeptide chains, enabling it to produce three effects when it binds oxygen, which makes Hb more suitable for transporting O2)
- 正协同效应(Positive cooperativity)
- 波尔效应(Bohr effect)
- 别构效应(Allosteric effect)

参考资料
- 南京大学 杨荣武老师 《结构生物学》课件
